Bovine AMH Testing
Introduction
Bovine Mullerian Inhibiting factor (UniProtKB-P03972), also known as anti-Mullerian hormone (AMH), is a glycoprotein encoded by the AMH gene (Gene ID: 280718, NCBI) located on chromosome 7 in cattle (Bos taurus). AMH is a member of the TGFβ Family of growth/differentiation factors (1). AMH determines sexual differentiation by inducing the regression of the Mullerian ducts in males during fetal development (and in a female twin of a male fetus resulting in a freemartin calf). AMH is also known to be an important growth factor/hormone within the ovary. Physiologically AMH is secreted by granulosa cells of developing ovarian follicles. (2;3) Patho-physiologically AMH is known to be secreted by granulosa-theca cell tumors. (4) AMH is also secreted in males by Sertoli cells of the testis.(5;6) However, the physiological role of AMH in the adult male is at best poorly understood currently.
Based on the physiology of AMH, the veterinary and management utility of AMH levels in female cattle is increasingly being assessed, particularly as reagents for quantitative measurement of AMH in peripheral circulation are becoming more widely available. Even at this early stage of investigation, AMH clearly has utility.
The value of AMH testing in veterinary use during embryo transfer programs involving high value animals is developing rapidly. While AMH physiology supports the potential utility of AMH levels in assessing present and/or future fertility in herd management, evidence is developing relatively slowly due to relatively high testing costs. AMH can be measured in serum or plasma any time in the estrous cycle. AMH levels also provide a direct measure of functional ovarian reserve and is highly correlated with antral follicle count (AFC) determined by ultrasound. Serum levels of AMH have been shown to be more repeatable than antral follicle counts by transrectal ultrasonography. (7-9) Declining AMH levels indicate a decline in fertility/fecundity. Not surprisingly given the cost-driven nature of commercial cattle operations, more is currently known about the utility of AMH testing in dairy herd management than for beef production applications.
As the database of AMH measurements in cattle increases and testing becomes more affordable, the role of AMH in “Marker Assisted Selection” can be fully assessed and valued. This is a rapidly progressing technology in which molecular and biochemical markers are combined with traditional phenotype assessments to select traits optimal for milk and dairy production as well as for cost effective herd management. For example, genotyping of variants of milk protein genes is currently being evaluated as a means of improving milk yield and/or composition. AMH testing offers potential for optimizing the prevalence of favorable genotypes in a herd by providing an objective tool for selecting the most fertile cows in a timely and cost-effective manner.

Serum AMH Levels: Potential Applications in Veterinary Medicine, Herd Management, and Bovine Research.
Predicting response to ovarian stimulation and embryo yield
Measuring AMH before enrolling cows in superovulation programs can be used to maximize the numbers of embryos successfully transferred, reducing the currently high costs per embryo produced.
AMH levels are directly related to the size of the antral follicular pool and is a powerful predictor of response to ovarian stimulation. AMH levels prior to ovarian stimulation are directly correlated with the resultant number of ovulations.(7;9-11)
AMH is also a marker of the quality of developing follicles. Healthy follicles at all stages of secondary and early antral development produce more AMH than unhealthy follicles. AMH has been shown to be a useful predictor of embryo yield. AMH concentration, measured in the plasma of donor dairy cows during first lactation and several months before the start of the embryo production campaigns, is highly correlated with the maximal number of recovered and transferable embryos per cow.(12)
Predicting fertility in dairy cows: selection for culling
AMH testing can contribute to improved profitability by reducing reproductive management costs.
This review focuses on dairy herds as the majority of what is known regarding the practical value of AMH testing has been obtained in studies of dairy cows, however the physiology of AMH appears to be virtually the same in dairy and beef cows of both subspecies of Bos Taurus (e.g. Bos taurus tarus and, Bos taurus indicus) as well as in cross-bred cattle. (13;14)
Reproductive management costs are often under-estimated and significantly impact profitability. Much of reproductive management depends on deciding which animals to cull and when. Fertility is a key trait used, along with other selection traits (such as milk production, sale values, etc.), considered in culling strategies. Fertility is assessed by sets of parameters that, in practice, do not distinguish fertility from fecundity and differ greatly across operators based on their experience, size of herd and operational strategies employed to maximize efficiency and/or profitability. Historically, the reproductive history of the cow or heifer in question and the reproductive history of the sire were the primary objective parameters. More recently genetic testing of cows may allow consideration of female genetic merit as well (note: sire traits for semen donor bulls have been available for many decades). Genetic trends for various traits (milk production, milk composition, productive life expectancy, daughter pregnancy rate, heifer and cow conception rates, calving ease, etc.) for several US dairy breeds are updated annually. Many models of calculating cost and returns are used by operators to design culling strategies. Generally, simple models do not include many significant factors that alter the balance between cull rate and how long a cow should be retained in the herd. The optimal combination of subjective and objective parameters has yet to be achieved to maximize profitability.(15) Adding AMH testing provides a unique and objective parameter to predict future reproductive potential based on ovarian function rather than genetics of the cow/heifer being evaluated.
As noted above, AMH testing provides an estimate of the number of ovarian follicles in adult cows. This is the basis for observations that AMH levels in adult cows are associated with:
– future easy of conception
– rate of pregnancy to term
– days in milk
– predicting response ovarian stimulation protocols, including number of ovulations and embryo yield
– predicting reproductive longevity in adult cows
AMH is also a powerful new tool in the selection of replacement heifers. Predicting the future reproductive rate of young cows as replacement for older cows whose production/reproductive performance has declined. AMH levels are associated with:
– predicting conception rates to Artificial Insemination
– predicting days open after calving for individual cows
– identifying, without compromising milk production, heifers with predicted overall superior reproductive performance
Each of these associations can, in principle, be adapted to the needs of specific operations as a significant aid in the process of deciding which animals to cull and when.
Diagnosis of Granulosa-Thecal Cell Tumors
AMH is a novel biomarker for granulosa-thecal cell tumors in cattle.
Neoplastic granulosa cells in surgical biopsy of granulosa-thecal cell tumors and processed for immunohistochemistry are clearly labeled with AMH antibody and plasma AMH levels are significantly higher in cows with granulosa-thecal cell tumors than healthy cows with a functional corpus luteum, cows with ovarian cysts or super-ovulated cows.(16)
Testing for the presence of functional testicular tissue.
AMH is the best marker for the presence of testicular tissue and/or Sertoli cell function.
Castrate bull (veterinary use) and Freemartin (research use) AMH testing is a cost-effective substitute for expensive surgical or imagining assessments.(5;6)
Basic research using bovine models of ovarian physiology and pathophysiology
AMH ELISAs provide an important new tool that is increasingly being utilized in basic research using bovine models to understand the physiology of AMH and early folliculogenesis as well as reproductive disorders of cattle and humans.
The cow has long been a model for the study of ovarian physiology and pathophysiology. The increasing understanding and use of embryo transfer technologies over the past several decades has contributed greatly as access to ovarian tissues are now widely available. Basic research using bovine models focuses on both improving our understanding of bovine disease and management and as a non-primate model of human ovarian function and fertility, especially at the molecular/cellular level. For example, assisted reproduction technologies such as ovarian stimulation, in-vitro transfer processes, and fertility preservation in humans were built on the knowledge gained from the development and application of bovine embryo transfers.
Currently bovine models offer significant advantage when using accurate and specific AMH measurements in blood, follicular fluid and/or culture medium to address basic questions regarding:
– The endocrine, paracrine and autocrine regulation of AMH biosynthesis and action (11;17)
– The dynamics of stage-specific AMH secretion during follicular maturation (3)
– The etiology of ovarian cyst formation, which is poorly understood, particularly at the intra-follicular or cellular level. Bovine cyst formation is likely driven by processes similar those that cause failure to ovulate in some women with PCOS and these processes are more easily studied in bovine models. (18)
Measurement of Bovine AMH
Measuring peripheral levels of AMH is currently, and most cost-effectively, achieved using double antibody immunoassay methods, primarily as an enzyme-linked immuno-absorbent assay (ELISA). Design of such methods critically influences the values obtained by and the utility of the testing. Assay designs, which vary considerably across current manufacturers of AMH ELISAs based on the structure and biological function of the hormone. The active hormone is a homodimer of the highly conserved region of the C-terminal of the secreted prohormone protein. Bovine and human AMH sequences are 78% homologous. The homodimer is covalently linked by interchain disulfides and, by analogy to the human hormone, is non-covalently associated with the protease cleaved N-terminal region of the pro-hormone which is the circulating and biologically active form of AMH. Key differences among AMH ELISAs involve the epitopes recognized by the antibodies used in the assay and by the source/purity of the assay calibrators. As such, results generated by ELISAs from different manufacturers are not commutable. Typically, measurements of the same specimens in different ELISAs are non-linearly related. Importantly, there is also a wide range of species-specificity and interfering materials across different ELISAs.
The first double-monoclonal bovine assay was developed using antibodies against AMH purified from bovine testes but lacked the sensitivity (i.e., the limit of detection was 20 ng/mL) to measure AMH in bovine serum or plasma except in bulls younger than 6 weeks of age. (19) Subsequent double monoclonal antibody assays, as summarized in Table 1 were designed to measure levels of AMH in adult women (0.1 to > 5 ng/mL) and lacked species specificity so they could be used to measure bovine AMH (purified or simply newborn bull serum). The first human assay used to measure AMH in serum or plasma from adult cattle was the Active AMH/MIS ELISA manufactured by Diagnostic Laboratory Systems, Inc (DSL) who was later acquired by Beckman-Coulter Diagnostics. The DSL assay was designed to measure AMH in human serum which was significantly lower than AMH levels in bovine serum, but as the assay’s limit of detection was 20 to 40 pg/mL it could, by increasing the specimen volume tested, measure AMH in most albeit not all (less than about 90%) bovine serum specimens.(11;18;20;21) Beckman replaced this assay with its Gen II Human AMH ELISA using the same monoclonal antibodies and basic assay design but the Gen II assay was less sensitive than the DSL assay and the values reported by this assay (bovine or human) are not commutable across the various re-calibrations and modified protocols that mark its history of use. Neither the DSL nor Gen II assay were ever rigorously validated for the measurement of bovine AMH. Assay performance and perhaps validity underlie at least part of the high variability of bovine AMH results reported using these assays.
The first bovine specific AMH assay is the Ansh Bovine AMH ELISA. This assay uses a unique pair of monoclonal antibodies. The assay is calibrated using recombinant bovine AMH and does not cross react with human AMH (< 0.001% based on protein mass). This assay is described in detail below and has been the mostly widely used to measure AMH in bovine serum/plasma since it became widely available in 2012. (7;8;22-24)
Measurement of bovine serum AMH using the ANSH ELISA (AL-114)
This assay was designed specifically for the measurement of bovine AMH in serum, plasma, whole blood, follicular fluid or culture media. The monoclonal antibodies are manufactured by Ansh Labs and are directed against the prohormone and mature (C-terminal) regions of the secreted protein; both covalent and non-covalent complexes of pro-mature AMH as it exists in circulation are measured specifically. The assay is calibrated (approximately 15 to 2,000 pg/mL) with recombinant bovine AMH and does not recognize human AMH or other TGFβ family members.
Rigorous validation of the Ansh Bovine AMH ELISA has been published. (8) The limit of detection for measuring AMH in bovine serum is 11 pg/mL which allows quantitative measurement of the low levels of AMH observed in adult cattle. The Ansh Labs’ Bovine AMH ELISA kit is manufactured in conformance with ISO13485:2016 standards (Ansh Labs is an ISO certified manufacturer).Because of its sensitivity, specific design for measuring bovine AMH, and manufacturing consistency, a majority of the peer-reviewed publications since 2014 reporting measurements of AMH in bovine serum/plasma have utilized this method.
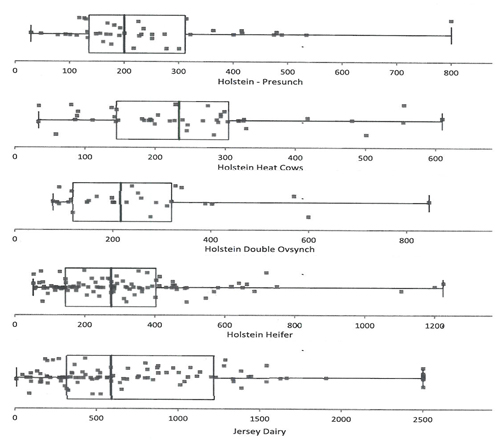
As with any laboratory test intended for diagnostic or management use, interpretation depends on normative or reference data. Figure 1 is a summary of serum AMH values acquired by Ansh Esoteric Laboratory testing services using the Ansh Bovine AMH ELISA.
Table 1: Bovine AMH Assays (Download)
Figure 1: AMH levels (pg/mL) in Cattle using the Ansh Bovine AMH ELISA
Reference List
1. Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP 1986 Isolation of the bovine and human genes for Mullerian inhibiting substance and expression of the human gene in animal cells. Cell 45:685-698
2. Ilha GF, Rovani MT, Gasperin BG, Ferreira R, de Macedo MP, Neto OA, Duggavathi R, Bordignon V, Goncalves PBD 2016 Regulation of Anti-Mullerian Hormone and Its Receptor Expression around Follicle Deviation in Cattle. Reprod Dom Anim 51:188-194
3. Yang MY, Cushman RA, Fortune JE 2017 Anti-Mllerian hormone inhibits activation and growth of bovine ovarian follicles in vitro and is localized to growing follicles. Mol Hum Reprod 2017/03/17:282-291
4. El-Sheikh Ali H, Kitahara G, Nibe K, Yamaguchi R, Horii Y, Zaabel S, Osawa T 2013 Plasma anti-Mullerian hormone as a biomarker for bovine granulosa-theca cell tumors: Comparison with immunoreactive inhibin and ovarian steroid concentrations. Theriogenology 80:940-949
5. Kitahara G, Kamata R, Sasaki Y, El-Sheikh Ali H, Mido S, Kobayashi I, Hemmi K, Osawa T 2016 Changes in peripheral anti-Mullerian hormone concentration and their relationship with testicular structure in beef bull calves. Domestic Animal Endocrinology 57:127-132
6. Scarlet D, Aurich C, Ille N, Walter I, Weber C, Pieler D, Peinhopf W, Wohlsein P, Aurich J+ 2017 Anti-Muellerian hormone, inhibin A, gonadotropins, and gonadotropin receptors in bull calves after partial scrotal resection, orchidectomy, and Burdizzo castration. Theriogenology 87:242-249
7. Souza AH, Carvalho PD, Rozner AE, Vieira LM, Hackbart KS, Bender RW, Dresch AR, Verstegen JP, Shaver RD, Wiltbank MC 2015 Relationship between circulating anti-Mullerian hormone (AMH) and superovulatory response of high-producing dairy cows. Journal of Dairy Science 98:169-178
8. Ribeiro ES, Bisinotto RS, Lima FS, Greco LF, Morrison A, Kumar A, Thatcher WW, Santos JEP 2014 Plasma anti-Mullerian hormone in adult dairy cows and associations with fertility. Journal of Dairy Science 97:6888-6900
9. Rico C, Drouilhet L, Salvetti P, Dalbies-Tran R, Jarrier P, Touze J-L, Pillet E, Ponsart C, Fabre S, Monniaux D 2012 Determination of anti-Mullerian hormone concentrations in blood as a tool to select Holstein donor cows for embryo production: from the laboratory to the farm. Reprod Fertil Dev 24:932-944
10. Abdel Aziz RL, Khalil AAY, Abdel-Wahab A, Hassan NY, Abdel-Hamied E, Kasimanickam RK 2017 Relationship among circulating anti-Mullerian hormone, insulin like growth factor 1, cadmium and superovulatory response in dairy cows. Theriogenology 100:72-79
11. Rico C, Medigue C, Fabre S, Jarrier P, Bontoux M, Clement F, Monniaux D 2011 Regulation of Anti-Mulerian Hormone Production in the Cow: A Multiscale Study at Endocrine, Ovarian, Follicular, and Granulosa Cell Levels. Biology of Reproduction 84:560-571
12. Monniaux D, Barbey S, Rico C, Fabre S, Gallard Y, Larroque H 2010 Anti-Mllerian hormone: a predictive marker of embryo production in cattle. Reprod Fertil Dev 22:1083-1091
13. Mossa F, Jimenez-Krassel F, Scheetz D, Weber-Nielsen M, Evans ACO, Ireland JJ 2017 Anti-Mullerian hormone (AMH) and fertility management in agricultural species. 154 ed.; R1-R11
14. Baruselli PS, Batista EOS, Vieira LM, Souza AH 2015 Relationship between follicle population, AMH concentration and fertility in cattle. 12 ed.; 487-497
15. De Vries A 2017 Economic trade-offs between genetic improvement and longevity in dairy cattle. Journal of Dairy Science 100:4184-4192
16. Kitahara G, Nambo Y, El-Sheikh Ali H, Kajisa M, Tani M, N K, Kamimura S 2012 Anti-Mullerian Hormone Profiles as a Novel Biomarker to Diagnose Granulosa-theca Cell Tumors in Cattle. Journal of Reproduction and Development 58:98-104
17. Crisosto N, Sir-Petermann T, Greiner M, Maliqueo M, Moreno M, Aedo P, Lara HE 2009 Testosterone-induced downregulation of anti-Mullerian hormone expression in granulosa cells from small bovine follicles. Endocrine 36:339-345
18. Monniaux D, Clemente N, Touze J-L, Belville C, Rico C, Bontoux M, Picard JY, Fabre S 2008 Intrafollicular Steroids and Anti-Mullerian Hormone During Normal and Cystic Ovarian Follicular Development in the Cow. Biology of Reproduction 79:387-396
19. Necklaws EC, LaQuaglia MP, MacLaughlin D, Hudson P, Mudgett-Hunter M, Donahoe PK 1986 Detection of Mullerian inhibiting substance in biological samples by a solid phase sandwich radioimmunoassay. Endocrinology 118:791-796
20. Arouche N, Picard JY, Monniaux D, Jamin SP, Vigier B, Josso N, Cate RL, di Clemente N, Taieb Jl 2015 The BOC ELISA, a ruminant-specific AMH immunoassay, improves the determination of plasma AMH concentration and its correlation with embryo production in cattle. Theriogenology 84:1397-1404
21. Rico C, Fabre S, Medigue C, Clemente N, Clement F, Bontoux M, Touze J-L, Dupont M, Briant E, Remy B, Beckers JF, Monniaux D 2009 Anti-Mullerian Hormone Is an Endocrine Marker of Ovarian Gonadotropin-Responsive Follicles and Can Help to Predict Superovulatory Responses in the Cow. Biology of Reproduction 80:50-59
22. Gobikrushanth M, Dutra PA, Bruinje TC, Colazo MG, Butler ST, Ambrose DJ 2017 Repeatability of antral follicle counts and anti-Mullerian hormone and their associations determined at an unknown stage of follicular growth and an expected day of follicular wave emergence in dairy cows. Theriogenology 92:90-94
23. Center K, Dixon D, Looney C, Rorie R 2018 Anti-Mullerian Hormone and Follicle Counts as Predictors of Superovulatory Response and Embryo Production in Beef Cattle. 6 ed.; 22-33
24. Arouche N, Picard JY, Monniaux D, Jamin SP, Vigier B, Josso N, Cate RL, di Clemente N, Taieb J 2015 The BOC ELISA, a ruminant-specific AMH immunoassay, improves the determination of plasma AMH concentration and its correlation with embryo production in cattle. Theriogenology 84:1397-1404
25. Ireland JLH, Scheetz D, Jimenez-Krassel F, Themmen APN, Ward F, Lonergan P, Smith GW, Perez GI, Evans ACO, Ireland JJ 2008 Antral Follicle Count Reliably Predicts Number of Morphologically Healthy Oocytes and Follicles in Ovaries of Young Adult Cattle1. Biology of Reproduction 79:1219-1225
26. HIRAYAMA H, KAGEYAMA S, NAITO A, FUKUDA S, FUJII T, MINAMIHASHI A 2012 Prediction of Superovulatory Response in Japanese Black Cattle Using Ultrasound, Plasma Anti-Mullerian Hormone Concentrations and Polymorphism in the Ionotropic Glutamate Receptor AMPA1/GRIA1. Journal of Reproduction and Development 58:380-383
27. Ghanem N, Jin JI, Kim SS, Choi BH, Lee KL, Ha AN, Song SH, Kong IK 2016 The Anti-Mullerian Hormone Profile is Linked with the In Vitro Embryo Production Capacity and Embryo Viability after Transfer but Cannot Predict Pregnancy Outcome. Reprod Dom Anim 51:301-310
28. Batista EOS, Macedo GG, Sala RV, Ortolan MDDV, Sa Filho MF, Del Valle TA, Jesus EF, Lopes RNVR, Renno FP, Baruselli PS 2014 Plasma Antimullerian Hormone as a Predictor of Ovarian Antral Follicular Population in Bos indicus (Nelore) and Bos taurus (Holstein) Heifers. Reprod Dom Anim 49:448-452
29. Guerreiro BM, Batista EOS, Vieira LM, S+í Filho MF, Rodrigues CA, Castro Netto A, Silveira CRA, Bayeux BM, Dias EAR, Monteiro FM, Accorsi M, Lopes RNVR, Baruselli PS 2014 Plasma anti-mullerian hormone: an endocrine marker for in-vitro embryo production from Bos taurus and Bos indicus donors. Domestic Animal Endocrinology 49:96-104
30. VERNUNFT A, SCHWERHOFF M, VIERGUTZ T, DIEDERICH M, KUWER A 2015 Anti-Muellerian hormone levels in plasma of Holstein-Friesian heifers as a predictive parameter for ovum pick-up and embryo production outcomes. Journal of Reproduction and Development 61:74-79
31. Jimenez-Krassel F, Scheetz DM, Neuder LM, Ireland JLH, Pursley JR, Smith GW, Tempelman RJ, Ferris T, Roudebush WE, Mossa F, Lonergan P, Evans ACO, Ireland JJ 2015 Concentration of anti-Mullerian hormone in dairy heifers is positively associated with productive herd life. Journal of Dairy Science 98:3036-3045
32. Gamarra G, Ponsart C, Lacaze S, Le Guienne B, Humblot P, Deloche MC, Monniaux D, Ponter AA 2015 Dietary propylene glycol and in vitro embryo production after ovum pick-up in heifers with different anti-Mullerian hormone profiles. Reprod Fertil Dev 27:1249-1261
33. Carter AS, Mahboubi K, Costa NN, Gillis DJ, Carter TF, Neal MS, Miranda MS, Ohashi OM, Favetta LA, King WA 2016 Systemic and local anti-Mullerian hormone reflects differences in the reproduction potential of Zebu and European type cattle. Animal Reproduction Science 167:51-58
34. Stojsin-Carter A, Costa NN, De Morais R, De Bem TH, Costa MP, Carter TF, Gillis DJ, Neal MS, Ohashi OM, Miranda MS, Meirelles FV, Favetta LA, King WA 2017 Fetal sex alters maternal anti-Mullerian hormone during pregnancy in cattle. Animal Reproduction Science 186:85-92
35. Kavya KM, Sharma RK, Jerome A, Phulia SK, Singh I 2017 Anti-Mullerian hormone and antral follicular count in early and delayed pubertal Murrah buffalo heifers. Livestock Science 198:89-92
36. HIRAYAMA H, NAITO A, FUKUDA S, FUJII T, ASADA M, INABA Y, TAKEDOMI T, KAWAMATA M, MORIYASU S, KAGEYAMA S 2017 Long-term changes in plasma anti-Mullerian hormone concentration and the relationship with superovulatory response in Japanese Black cattle. Journal of Reproduction and Development 63:95-100
37. Garcia-Guerra A, Motta JCL, Melo LF, Kirkpatrick BW, Wiltbank MC 2017 Ovulation rate, antral follicle count, and circulating anti-Mullerian hormone in Trio allele carriers, a novel high fecundity bovine genotype. Theriogenology 101:81-90
38. Nawaz MY, Jimenez-Krassel F, Steibel JP, Lu Y, Baktula A, Vukasinovic N, Neuder L, Ireland JLH, Ireland JJ, Tempelman RJ 2018 Genomic heritability and genome-wide association analysis of anti-Mullerian hormone in Holstein dairy heifers. Journal of Dairy Science 101:8063-8075
39. Maculan R, Pinto TLC, Moreira GM, de Vasconcelos GL, Sanches JA, Rosa RG, Bonfim RR, Goncalves TM, de Souza JC 2018 Anti-Mullerian Hormone (AMH), antral follicle count (AFC), external morphometrics and fertility in Tabapua cows. Animal Reproduction Science 189:84-92
40. Gobikrushanth M, Purfield DC, Colazo MG, Butler ST, Wang Z, Ambrose DJ 2018 The relationship between serum anti-Mullerian hormone concentrations and fertility, and genome-wide associations for anti-Mullerian hormone in Holstein cows. Journal of Dairy Science 101:7563-7574
41. Akbarinejad V, Gharagozlou F, Vojgani M, Bagheri Amirabadi MM 2018 Nulliparous and primiparous cows produce less fertile female offspring with lesser concentration of anti-Mullerian hormone (AMH) as compared with multiparous cows. Animal Reproduction Science 197:222-230
42. Redhead AK, Siew N, Lambie N, Carnarvon D, Ramgattie R, Knights M 2018 The relationship between circulating concentration of AMH and LH content in the follicle stimulating hormone (FSH) preparations on follicular growth and ovulatory response to superovulation in water buffaloes. Animal Reproduction Science 188:66-73
43. Akbarinejad V, Gharagozlou F, Vojgani M, Shourabi E, Makiabadi MJM 2019 Inferior fertility and higher concentrations of anti-Mullerian hormone in dairy cows with longer anogenital distance. Domestic Animal Endocrinology 68:47-53
44. Gobikrushanth M, Purfield DC, Kenneally J, Doyle RC, Holden SA, Martinez PM, Canadas ER, Bruinje TC, Colazo MG, Ambrose DJ, Butler ST 2019 The relationship between anogenital distance and fertility, and genome-wide associations for anogenital distance in Irish Holstein-Friesian cows. Journal of Dairy Science 102:1702-1711